TARGET POPULATION
NUMBER OF ITEMS
TIME TO COMPLETE
minutes
FORMAT
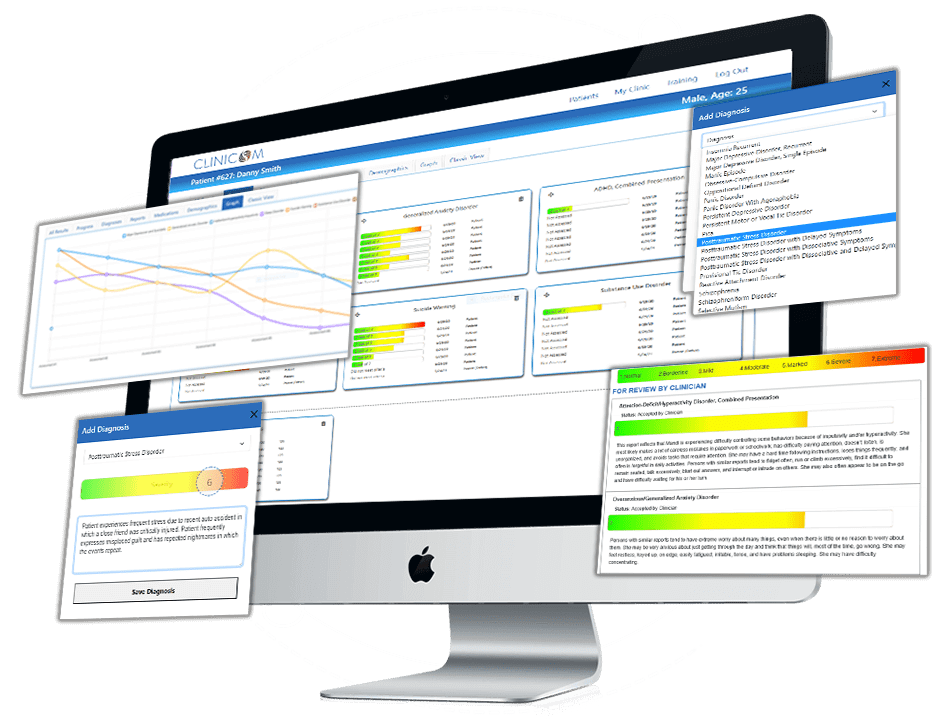
The mental health assessment mentioned here may or may not be included in Clinicom’s platform. However, Clinicom offers a dynamic adaptive assessment for 81 DSM conditions. Clinicians, start your free trial today.
Clinicians, learn how you can get a Free Trial to Clinicom and start assessing your patients with assessments that adapt to your patients needs unlike any other assessment in history.
Important Note for Patients: